Date: 04-19-03 23:27
THE ECCRINE SWEAT GLAND AS A POSSIBLE FOCUS OF INFECTION WITH ACID-FAST CELL-WALL-DEFICIENT BACTERIA
Alan R. Cantwell Jr., M.D.
Los Angeles, CA
ABSTRACT
Intra-eccrine acid-fast granules and larger acid-fast forms (“large bodies”) were seen in a case of linear scleroderma; in two cases of cutaneous lupus erythematosus; in a case of chronic myelogenous leukemia with dermatitis; and in a case of mycosis fungoides (also known as T-cell lymphoma of the skin). These acid-fast forms were observed by use of specific acid-fast staining techniques designed for the demonstration of acid-fast cell-wall-deficient (CWD) bacteria. The possible relationship between these sweat gland findings and the current finding of CWD bacteria in human blood is discussed, as well as the speculative role of CWD bacteria as causative agents in certain autoimmune, lymphoproliferative, and neoplastic diseases.
INTRODUCTION
During the course of investigations for acid-fast bacteria in panniculitis and scleroderma (1-6), the presence of variably acid-fast “granular” material was noted within some, but not all, eccrine sweat glands. At present, histopathologists consider eccrine acid-fast granules as a normal finding (7).
Cantwell and Kelso also observed variably acid-fast coccoid forms in tissue sections of the heart, adrenal gland, connective tissue and skin in necropsy material from a fatal case of systemic scleroderma (8) . They speculated that these eccrine granules might represent cell-wall-deficient (CWD) bacteria.
The role of CWD bacteria in health and disease is controversial. Studies by Wuerthele-Caspe Livingston and Livingston ( 9 ), Tedeschi et al. (10), and Domingue et al. (11), have all suggested that human blood may harbor CWD bacteria which on culture may “revert” to micrococci, diphtheroid-like bacilli (corynebacteria), and streptococcal-like organisms. Some of the bacteria cultured from blood cells by Tedeschi et al. were acid-fast (12 ), a feature shared by bacteria observed and cultured from certain neoplastic and immunologic diseases. Very recent studies by Brown et al. (13 ), and McLaughlin et al. (14 ), suggest that latent hematologic infection with CWD bacteria may be universal.
In this regard, human sweat is basically a hypotonic filtrate of the blood. Consequently, CWD infection of the blood may filter over into the sweat glands. Sweat abnormalities frequently accompany infection. In addition, variably acid-fast CWD microbes have been reported in association with cancer, lymphoproliferative disease, mycosis fungoides, sarcoidosis, and autoimmune diseases. See Mattman (15) and Cantwell (16-17) for an extensive review of this literature.
Could the presence of intra-eccrine acid-fast forms relate to the finding of bacteria in a wide variety of disease states, or to possible hematologic “crypto-infection”, or both? CWD bacteria are characterized by their variable size, ranging from sub-microscopic virus-like forms up to giant “large bodies”, as described by Dienes (18 ). CWD forms stain poorly, if at all, with Gram’s stain.
Mattman has proposed special modified acid-fast histologic staining techniques to demonstrate acid-fast CWD bacteria, which were utilized in this study. In this report, acid-fast eccrine forms were observed in skin biopsy material from a case of linear scleroderma (morphea), two cases of cutaneous lupus erythematosus, a case of mycosis fungoides, and a case of chronic myelogenous leukemia with dermatitis. An attempt is made to correlate the appearance of acid-fast eccrine forms with similar forms seen elsewhere in the dermis and panniculus of the skin, as well as in other organs.
MATERIAL AND METHODS
In this study, special acid-fast staining techniques, specifically designed for the detection of CWD bacteria in tissue microsopic sections, were employed, These included the Intensified Kinyoun stain, as recommended by Mattman ( 19), and the Alexander-Jackson triple stain for mycobacteria (20 ), modified by Hauderoy and Tanner (21). Routine acid-fast stains such as the Ziehl-Neelson, Fite, Fite-Faraco, and regular Kinyoun stain, were also employed. Forms, which were acid-fast (red, pink) when special staining techniques were employed, were often non-acid-fast (blue, purple) with regular acid-fast staining techniques. Prolonged immersion of the tissue sections in Kinyoun’s stain for 24 to 36 hours undoubtedly allowed better penetration and retention of the basic fuchsin.
Control material included acid-fast stained tissue sections from skin biopsy of 6 cases of dermatitis. Clinically, these cases were diagnosed as insect bite reaction, miliaria, and other rapidly clearing inflammations. No acid-fast eccrine material was observed in these cases.
Based on personal observations of acid-fast stained eccrine glands studied in over 100 different clinical cases with assorted pathologic processes, it is concluded that eccrine acid-fast “granules” are a “non-specific finding.” They may be found in a variety of pathologic conditions which are not included in this report. The five cases chosen exhibit certain acid-fast sweat gland findings which can serve as a basis for further investigation into the origin and significance of eccrine granules. In this sense, this report can be considered as “preliminary.”
REPORT OF CASES:
CASE 1:
In August 1977, a nine year old Mexican-American girl presented with a linear lesion of scleroderma extending from the lateral aspect of the left foot up to the thigh. The lesion was first observed on the foot two years previously. There was no history of trauma, and the patient was otherwise in good health. Neurologic and radiographic examination of the leg was normal. Laboratory investigation was unremarkable except for a reactive ANA titer of 1:80 (homogeneous pattern).
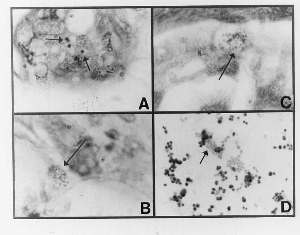
Figure 1 (click to enlarge)
A 3-mm punch biopsy specimen showed dense collagenous bands within the dermis, a mild deep dermal perivascular infiltrate, and atrophy of the skin appendages. These features were consistent with scleroderma. Acid-fast granular material was noted in some eccrine glands in sections stained with the Alexander-Jackson stain (Fig. 1C). Culture for bacteria in thioglycollate broth yielded a slow-growing, variably-sized coccus compatible with Staphylococcus epidermidis (Fig. 1D).
A comparison of the form and size of the cultured cocci with the form and size of the eccrine granules (and the dermal coccoid forms) all suggest a similarity. Highly pleomorphic, acid-fast bacteria have been reported to be associated with systemic scleroderma and localized scleroderma (morphea) by Wuerthele-Caspe (Livingston) et al. ( 22,23 ), Delmotte and Van der Meiren ( 24 ), and Cantwell et al. ( 2,3,4,5,6, 8).
CASE 2:
A black man, born in 1908, developed progressive scarring alopecia of the scalp in 1955. In 1978, he presented with multiple, scarred lesions with erythematous, scaling borders and follicular plugging involving the scalp, ears, and preauricular areas, Past history revealed hypertension. Abnormal laboratory findings included a mild leucopenia on 3 occasions of 3500/4000 cu mm, and an elevated IgG level (2250 mg.dL; normal, 800-16000 mg/dL). IgA and IgM levels were normal. The tuberculin skin test (250 units) was reactive.
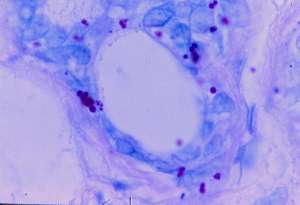
Figure 2 (enlarge)
Skin biopsy of an active border of a scalp lesion showed liquifactive degeneration of the basal layer and a perivascular and periappendageal infiltrate composed primarily of lymphocytes. These findings were consistent with lupus erythematosus. Sections stained with the Intensified Kinyoun stain showed small, acid-fast eccrine forms, some of which were single and others which appeared clumped together (Fig. 2).
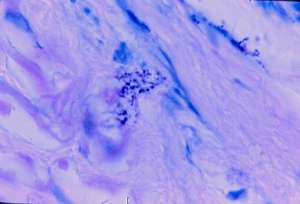
Fig.3 (enlarge)
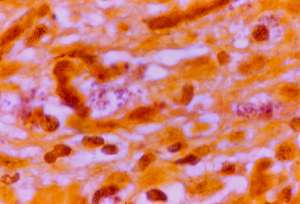
Fig.4 (enlarge)
Very rare similar sized but paler staining acid-fast coccoid forms were detected in the deep dermis (Fig.3). In the center of Fig. 4, a nest of pink-staining coccoid forms was observed in the upper dermis, by use of the Alexander-Jackson stain. These forms were barely visible, and stained faintly eosinophilic (pink) on routine hematoxylin-eosin stain (Fig. 5).
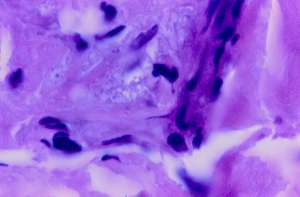
Fig.5 (enlarge)
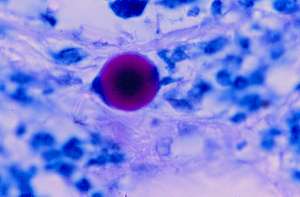
Fig.6 (enlarge)
A rare giant, acid-fast large body was observed in the mid-dermis (Fig 6). These forms are many times larger than a red blood cell and can easily be overlooked or ignored as an “artifact.” However, giant large bodies are well-known to microbiologists and this form is very similar to the appearance of bona-fide large bodies, or so-called “giant L-forms.” These forms also may bear some similarity to the “L.E. cell” found in the blood.
Culture of the lesion for bacteria was positive for a slow-growing, non-acid-fast, Gram positive, club-shaped, corynebacteria-like bacillus, identified as Propionibacterium acnes (Fig.7). Schaumann and Introzzi isolated a similar appearing diphtheroid organism (depicted in their Table 4, figure 8,9) from the blood of a fatal case of systemic lupus erythematosus. Their isolate produced tuberculosis on inoculation into guinea pigs (25 ).
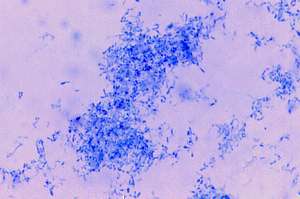
Fig.7 (enlarge)
CASE 3:
A black woman, born in 1931, developed cutaneous lupus erythematosus of the scalp in 1966, characterized by scarring alopecia and follicular plugging. In April 1978, she was noted to have 4 distinct lesions, the largest of which was palm-sized. The borders of the lesions were erythematous and hyperpigmented. Past history revealed treatment for syphilis in 1959. A complete blood count showed a mild leucopenia of 3,700 cu/mm, a hemoglobin level of 11.3 g/dL, and an erythrocyte sedimentation rate (Wintrobe) of 39 mm/hr. The ANA titre, lupus erythematosus cell preparation, and latex fixation test for rheumatoid factor were all normal. A VDRL reported in 1978 was normal; but had been reported as weakly reactive in 1977.
A skin biopsy taken from the active border of the scalp lesion revealed an atrophic epidermis, edema of the upper cutis, and a perivascular and periappendageal infiltrate composed primarily of lymphocytes. There was a dense infiltrate composed primarily of lymphocytes. There was a dense infiltrate around the atrophic hair follicle, and around the sweat ducts. The histologic findings were consistent with lupus erythematosus. Culture of the lesion for bacteria was negative.
Sections stained with the Intensified Kinyoun stain showed rare, intra-eccrine, large, solid-staining, acid-fast forms, as well as rare, large, clear forms with a rim of acid-fast staining material (Fig. 8 A,B). In addition, rare, smaller, acid-fast granular forms were present. These large forms, some of which were larger than erythrocytes (Fig. 8 C), were suggestive of “large bodies,” as previously described in a case of scleroderma associated with Mycobacterium fortuitum ( 2,3,6), (Fig. 8 D).
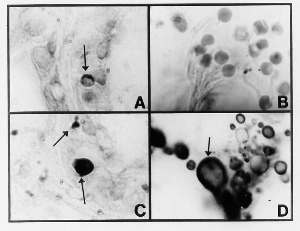
Fig.8 (enlarge)
CASE 4:
In December 1978, a 76 year old white man was seen for a pruritic, erythematous, and urticarial-like eruption primarily confined to the anterior portion of the trunk. The eruption had been intermittent for the previous 5 years, but was confined to a few patches of mildly pruritic dermatitis of the lower part of the abdomen. However, in June 1978, the patient was diagnosed with chronic myelogenous leukemia (Philadephia chromosome negative). At the time the leucocyte count was markedly elevated to 72,000 cu/mm with 90% segmented neutrophils. The dermatitis had markedly worsened, becoming more urticarial in appearance during chemotherapy with bisulfan.
A skin biopsy showed a mild cellular infiltrate composed of lymphocytes, some with large vesicular nuclei which were seen affecting the upper dermis, particularly in a perivascular distribution. A few neutrophils were also present, but mitotic figures were not seen. The histologic diagnosis was “non-specific inflammation of the skin” (Fig. 9A). Histologic staining with the Intensified Kinyoun stain and the Alexander-Jackson triple stain for mycobacteria revealed large numbers of acid-fast coccoid forms in some, but not all, eccrine glands. Rare, large, intra-eccrine froms suggested the appearance of the large body (Fig. 9 C,D). Culture of the lesion was not performed.
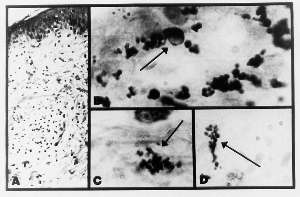
Fig.9 (enlarge)
Despite chemotherapy, the patient experienced a downhill course aggravated by urate nephropathy, stomach ulceration, and ascitis. In December 1979, an abdominal exploration was performed due to suspicion of carcinoma of the stomach. None was found. The patient died in January 1980.
Interestingly, acid-fast bacteria have been associated with leukemia, as reported by Diller and Diller (26,27), Mazet (28,29 ), and Seibert et al. (30,31 ).
CASE 5:
A 52 year old Japanese-American man with the clinical and histologic diagnosis of mycosis fungoides (T cell lymphoma) died in 1976. He had had a 9 year history of eczematous and pruritic dermatitis. One year prior to death, he developed marked inguinal lymphadenopathy, pedal edema, and inflammatory cell tumefactions of the skin with non-healing ulcerations. Chemotherapy with prednisone, methotrexate, nitrogen mustard and vincristine was ineffective. During the last month of his life he was hospitalized for severe swelling of the tonsillar area and dysphagia, along with increasing weakness, shortness of breath, and persistent fever. Numerous blood cultures were negative, except one which showed Pseudomonas maltophilia which was regarded as a contaminant. Despite vigorous antibiotic therapy, the patient died.
The final diagnosis at necropsy was termed “Diffuse, poorly differentiated lymphoma, stage IV.” All the available skin biopsy and necropsy material was studied for the presence of acid-fast bacteria in histologic skin sections.
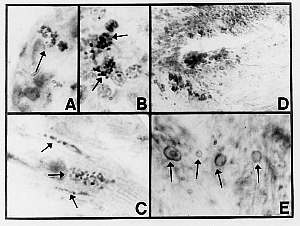
Fig.10 (enlarge)
Sections of a skin biopsy specimen taken 4 years before the patient’s death showed foci of acid-fast intra-eccrine coccoid forms (Fig. 10 A). Very rare, similar stained forms were demonstrated in an inguinal lymph node excised one year before the patient’s death, and reported as “moderately well differentiated diffuse lymphocytic lymphoma” (Fig. 10 B).
At necropsy, similar acid-fast forms were detected in the myocardium (Fig. 10 C). Structures resembling large bodies were identified within the mitral valve sections stained with hematoxylin-eosin (Fig. 10 D). The gross pathologic description of the valve area was normal, but the microscopic description stated that “the mitral valves show some hyalinization.” The gross and microscopic examination of the heart musculature was recorded as normal. Some of the basophilic-rimmed large bodies appeared to contain basophilic nuclear-like material (Fig. 10 E). Moscovic (32) has labelled similar forms seen in lymph nodes in sarcoidosis as “ring forms” with “volutin granules” (see his figure 6), which are characteristic forms of “type 3, mycobacterial pleomorphic chromogens.” The occurrence of mycobacterial forms within the heart was reported by Wuerthele-Caspe Livingston and Alexander-Jackson in 1965 ( 33).
DISCUSSION
There have been very few reports concerning eccrine granules. They are poorly visualized, if at all, in routine hematoxylin-eosin stained tissue sections, and their occasional presence in pathologic material has been largely ignored. Dobson and Abele (34 ) described globular or irregularly-shaped, 1-3 micron size, blue-green, Giemsa-stained particles in eccrine glands of hypothyroid patients. These particles were not present in euthyroid or other “normal” individuals. Utilizing the Nile –blue stain, Cawley et al. studied dark blue and blue-green eccrine granules in 30 individuals, aged 2 to 60, at necropsy (35 ). By use of the light microscope, granules were observed in all cases, except those under the age of 12. They considered the granules to be lyzosomes. Moscovic studying similar acid-fast structures in lymph nodes (but not sweat glands) in sarcoidosis, has interpreted these forms as mycobacterial L-bodies (i.e. CWD bacteria forms) (32 ). Moscovic notes the current controversy between the two opposing concepts of ceroid and lipofuchsin (“wear and tear”) pigment versus mycobacterial elements.
By use of the Giemsa stain, certain CWD bacteria may be visualized in histologic sections as Giemsa-positive coccoid forms. However, in this study more specific acid-fast staining techniques were employed which allowed differential staining of microbes to a greater degree than regular acid-fast tissue stains. For example, both the Intensified Kinyoun stain and the modified Alexander-Jackson triple stain require that the sections be immersed in carbol-fuchsin for at least 24 hours, unlike regular acid-fast staining which requires only a few minutes (Ziehl-Neelson stain) or up to one hour (regular Kinyoun stain).
In case 1 (linear scleroderma), the varying size of the eccrine granules is strikingly similar to the varying size of the cocci identified as Staphylococcus epidermidis, cultured from the skin lesion (Fig. 1A,D). Collections of similar sized non-acid-fast coccoid forms were visible in the deep dermis by use of the Fite stain (Fig 1B,C). Interestingly, Cawley et al. were unable to find eccrine granules in cases under the age of 12 ( 35). This patient was only nine years old at the time of biopsy.
In case 2 (cutaneous lupus erythematosus), very rare, single and clumped, acid-fast coccoid forms were seen in the upper dermis by utilization of the Intensified Kinyoun stain (Fig 2B). These forms were similar to those seen in the eccrine gland (Fig 2A) except they were less strongly acid-fast (i.e. pink staining) (Fig 2B). Culture of the lesion was positive for a slow-growing, non-acid-fast corynebacterium-like (diphtheroid) organism.
Case 3, also cutaneous lupus erythematosus, exhibited very rare and highly unusual large, solid staining, acid-fast structures, as well as large, clear, acid-fast rimmed bodies within the eccrine gland (Fig 3A,B). These forms are morphologically similar to “large body” forms of CWD bacteria described by Dienes (18), Alexander- Jackson (36), Mattman et al. (37, 38) , Xalabarder (39 ), and Cantwell et al. (5, 6 ). The large intra-eccrine forms depicted in case 3 can hardly be interpreted as “granules” as that term ordinarily connotes a smaller, solid-staining form. Further details pertaining to CWD bacterial infection in cutaneous and systemic lupus erythematosus can be found in later papers by Cantwell (40), and Cantwell and Cove ( 41 ).
Case 4, non-specific dermatitis associated with myelogenous leukemia, again illustrates the variation of eccrine granules which range in size from small, acid-fast coccoid forms up to larger, clear, acid-fast rimmed bodies similar in size to “large bodies” (Fig 4B). Within the dermis and panniculus, rare foci of acid-fast coccoid forms were present, which resembled the intra-eccrine coccoid forms (Fig. 4C,D). The patient died of his disease one year after this biopsy was performed.
Case 5 (mycosis fungoides/ T cell lymphoma) illustrates intra-eccrine acid-fast forms (Fig. 5A) which are similar to the acid-fast coccoid forms observed in the lymph node (Fig. 5B) and in the cardiac muscle at death (Fig. 5C). Structures resembling large bodies were present in the area of the mitral valve (Fig. 5D.E). These rimmed bodies with occasional nuclear-like inclusions bear a strong resemblance to the large, clear, intra-eccrine forms depicted in case 3 and 4 (Fig. 3B and 4B), and in the case of scleroderma (Fig. 3D). The coccoid forms in the heart may have some relationship to so-called “lipofuchsin granules” in atrial cardial tissue, as described by Jamieson and Palade ( 42 ). Acid-fast bacteria in mycosis fungoides have been demonstrated by Aplas, who also cultured a virus-like microbe from this lymphoma which was pathogenic for mice (43, 44 ). Further autopsy findings of pleomorphic bacteria in case 5 were reported by Cantwell in 1982 (45).
Further observations and histologic study of acid-fast eccrine granules are needed to determine the full significance of these forms, especially in diseases of unknown etiology. This study suggests that eccrine forms are similar in morphology to microbial forms currently designated as CWD bacteria (L forms, protoplasts, spheroplasts, bacterial variants,etc.).
At present, the precise role of CWD bacteria in health and in disease has not been established. It is possible that the presence of acid-fast granules in eccrine sweat glands might provide a clue to possible infection with CWD bacteria.
LEGEND FOR FIGURES

FIGURE1: (View it full size
A) Section from linear scleroderma showing acid-fast eccrine granules (Case1; modified Alexander-Jackson stain; magnification x1000, in oil).
B) Section from scleroderma showing a rare focus of non-acid-fast coccoid forms in the deep dermis ( Case 1; Fite stain, magnification x1000, in oil).
C) Section from scleroderma showing a rare focus of acid-fast coccoid forms in the deep dermis (Case1; modified Alexander-Jackson stain; magnification x1000, in oil).
D) Smear from culture of non-acid-fast Staphylococcus epidermidis isolated in thioglycollate broth from linear scleroderma (Case 1: Ziehl-Neesen stain, magnification x1000, in oil.

FIGURE2: (View it full size)
Section of cutaneous lupus erythematosus showing single and clumped intra-eccrine acid-fast coccoid forms. (Case 2; Intensified Kinyoun stain; magnification x1000, in oil).

FIGURE3: (View it full size)
Same section as Fig 2, showing very rare, weakly acid-fast coccoid forms in the deep dermis, (Case 2; Intensified Kinyoun stain, magnification x1000, in oil).

FIGURE4: (View it full size)
Section of cutaneous lupus erythematosus showing (in center) a small focus of weakly acid-fast coccoid forms in the upper dermis. (Case 2; Alexander-Jackson triple stain, magnification x 1000, in oil).

FIGURE5: (View it full size)
Section of cutaneous lupus erythematosus showing barely visible (eosinophilic) coccoid forms in the mid-dermis with “routine” hematoxylin-eosin stain. (Case 2; magnification x 1000, in oil).

FIGURE6: (View it full size)
Section of cutaneous lupus erythematosus showing an acid-fast giant “large body” . Such structures are bona-fide growth forms of CWD bacteria. (Case 2; Intensified Kinyoun stain; magnification x 1000, in oil.

FIGURE7: (View it full size)
A) Smear of culture isolated in thioglycollate broth showing corynebacteria identified as Propionobacterium acnes. (Case 2; Ziehl-Neelsen stain; magnification x1000, in oil).
B) Same culture as (7.A) showing Gram-positive bacillus (Case 2; Gram stain, magnification x1000, in oil).

FIGURE8: (View it full size)
A) Section of cutaneous lupus erythematosus showing an eccrine gland which contains a large, crescent-shaped, acid-fast structure (Case 3; Intensified Kinyoun stain; magnification x1000, in oil).
B) Same section as (A) showing a solid-staining, acid-fast large body, and much smaller conventional-sized acid-fast granules (Case 3; magnification x1000, in oil).
C) Erythrocytes (red blood cells) in histologic section. Compare the size of these forms with the large bodies observed in Fig. A.B,C. (Hematoxylin-eosin stain, magnification x1000, in oil.
D) Section of scleroderma associated with Mycobacterium fortuitum showing basophilic-rimmed large bodies in the panniculus (fat). Compare with similar bodies in Fig. A,B. (Hematoxylin-eosin stain, magnification x1000, in oil.

FIGURE9: (View it full size)
A) Section of non-specific dermatitis associated with chronic myelogenous leukemia (Case 4; hematoxylin-eosin stain; magnification x 100).
B) Acid-fast coccoid forms and a large body (arrow) in an eccrine gland (Case 4; Intensified Kinyoun stain, magnification x1000, in oil).
C) Similar acid-fast coccoid forms in the mid-dermis (Case 4; Intensified Kinyoun stain; magnification x1000, in oil).
D) Similar acid-fast forms in the panniculus (Case 4; Intensified Kinyoun stain; magnification x1000, in oil).

FIGURE10: (View it full size)
A) Section of mycosis fungoides showing intra-eccrine acid-fast coccoid forms (Case 5; Intensified Kinyoun stain; magnification x1000, in oil).
B) Section of lymph node showing lymphoma and a rare focus of acid-fast coccoid forms similar to (A), (Case 5; Intensified Kinyoun stain; original magnification x1000, in oil).
C) Section of myocardium showing acid-fast coccoid forms (Case 5; Intensified Kinyoun stain; magnification x1000, in oil).
D) Section of mitral valve showing collections of basophilic-rimmed bodies suggestive of large bodies (Case 5; Hematoxylin-eosin stain; magnification x 400).
E) Oil immersion view of bodies seen in (D) showing enlarged forms with internal “volutin granules” and “ghost” forms (Case 5; hematoxylin-eosin stain; magnification x1000).
REFERENCES:
1. Cantwell AR Jr, Craggs E, Swatek F, Wilson JW. Unusual acid-fast bacteria in panniculitis. Arch Dermatol. 1966 Aug;94(2):161-7(Pubmed Abstract)
2. Cantwell AR Jr, Wilson JW. Scleroderma with ulceration secondary to atypical mycobacteria. Arch Dermatol. 1966 Nov;94(5):663-4(Pubmed Abstract)
3. Cantwell AR Jr, Craggs E, Wilson JW, Swatek F: Acid-fast bacteria as a possible cause of scleroderma. Dermatologica. 1968;136(3):141-50(Pubmed Abstract)
4. Cantwell AR, Kelso DW. Acid-fast bacteria in scleroderma and morphea. Arch Dermatol 1971; 104;21-25.
5. Cantwell AR Jr, Kelso DW, Rowe L. Hypodermitis sclerodermiformis and unusual acid-fast bacteria. Arch Dermatol. 1979 Apr;115(4):449-52(Pubmed Abstract)
6. Cantwell AR Jr. Histologic forms resembling "large bodies" in scleroderma and "pseudoscleroderma". Am J Dermatopathol. 1980 Fall;2(3):273-6(Pubmed Abstract)
7. Pinkus H, Mehregan AH. A Guide to histopathology, 2nd ed. New York: Appleton-Centruy-Crofts; 1976, p. 46.
8. Cantwell AR Jr, Kelso DW. Autopsy findings of nonacid-fast bacteria in scleroderma. Dermatologica. 1980;160(2):90-9(Pubmed Abstract)
9. Livingston V, Livingston AM. Demonstration of Progenitor cryptocides in the blood of patients with collagen and neoplastic diseases. Trans NY Acad Sci 1972; 174 (2):636-654(Pubmed Abstract)
10, Tedeschi GG, Sprovieri G, Del Prete P. Cocci and diphtheroids in blood cultures from patients in various pathological situations. Experientia. 1978 May 15; 3(5):5968(Pubmed Abstract)
11. Domingue GJ, Schlegal JU, Woody HB, et al. Naked bacteria in human blood. A novel concept for etiology of certain kidney diseases. Microbia 1976; 2:3-31
12. Tedeschi GG, Bondi A, Paparelli M, Sprovieri G. Electron microscopical evidence of the evolution of corynebacteria-like microorganisms within human erythrocytes. Experientia. 1978 Apr 15;34(4):458-60(Pubmed Abstract)
13. Brown ST, Brett I, Almenoff PL, Lesser M, Terrin M, Teirstein AS. Recovery of cell wall-deficient organisms from blood does not distinguish between patients with sarcoidosis and control subjects. Chest 2003 Feb;123(2):413-417(Pubmed Abstract)
14. McLaughlin RW, Vali H, Lau PC, Palfree RG, De Ciccio A, Sirois M, Ahmad D, Villemur R, Desrosiers M, Chan E. Are there naturally occurring pleomorphic bacteria in the blood of healthy humans? J Clin Microbiol. 2002 Dec;40(12):4771-5(Pubmed Abstract)
15. Mattman LH. Microbes and malignancy. In: Mattman LH. Cell wall deficient forms; Stealth pathogens, 2nd ed. Boca Raton: CRC Press; 1993. Pp. 311-321.
16. Cantwell AR. Variably acid-fast cell wall-deficient bacteria as a possible cause of dermatologic disease. In, Domingue GJ, editor. Cell wall deficient bacteria. Reading: Addison-Wesley Publishing Co; 1982. Pp. 321-360
17. Cantwell A. The Cancer microbe. Los Angeles: Aries Rising Press; 1990.
18. Dienes L. Morphology and reproductive processes of bacteria with defective cell wall. In, Guze LB, ed. Microbial protoplasts, spheroplasts, and L-forms. Baltimore: Williams and Wilkins; 1968. Pp 74-93.
19. Mattman LH. Media, methods, and stains. In: Mattman LH. Cell wall deficient forms; Stealth pathogens, 2nd ed. Boca Raton: CRC Press; 1993. Pp. 329-355.
20. Alexander-Jackson E. A differential triple stain for demonstrating and studying non-acid-fast forms of the tubercle bacillus in sputum, tissue, and body fluids. Science 1944; 99:307-308
21. Hauderoy P, Tanner F. Une coloration differentielle des Mycobacteries: la coloration d’Alexander. C R Soc Biol 1948; 142:1510-1511.
22. Wuerthele-Caspe (Livingston) V, Brodkin E, Mermod C. Etiology of scleroderma, a preliminary report. J Med Soc NJ 1947; 44(7);256-259.
23. Wuerthele Caspe-Livingston V, Alexander-Jackson E, Anderson JA, et al. Cultural properties and pathogenicity of certain microorganisms obtained from various proliferative and neoplastic diseases. Amer J Med Sci 1950; 220;628-646
24. Delmotte N, van der Meiren L. Recherches bacteriologiques et histologiques concernant la sclerodermie. Dermatologica 1953; 107;177-182
25. Schaumann J, Introzzi P. Sulla eziologia e sulla alterazioni sistematiche degli organi ematopoietici del lupus eritematoso acuta. Haematologica 1931; 12 (fasc 7);635-717.
26. Diller IC. Growth and morphological variability of pleomorphic, intermittently acid-fast organisms isolated from mouse, rat, and human malignant tissues. Growth 1962; 26:181-209.
27, Diller IC, Diller WF. Intracellular acid-fast organisms isolated from malignant tissues. Trans Amer Micr Soc 1965; 84:138-148(Pubmed Abstract)
28. Mazet G, Presence d’elements alcoolo-acido-resistants dans les moelles leucemiques et les moelles non-leucemiques. Semaine des Hopitaux 1962; No 1 et 2:35-38.
29. Mazet G. Corynebacterium, tubercle bacillus and cancer. Growth 1974; 38:61-74(Pubmed Abstract)
30. Seibert FB, Farrelly FK, Shepherd CC. DMSO and other combatants against bacteria isolated from leukemia and cancer patients. Ann N Y Acad Sci. 1967 Mar 15;141(1):175-201(Pubmed Abstract)
31. Seibert FB, Feldmann FM, Davis RL, Richmond IS: Morphological, biological, and immunological studies on isolates from tumors and leukemic bloods. Ann N Y Acad Sci. 1970 Oct 30;174(2):690-728(Pubmed Abstract)
32. Moscovic EA: Sarcoidosis and mycobacterial L-forms. A critical reappraisal of pleomorphic chromogenic bodies (Hamazaki corpuscles) in lymph nodes. Pathol Annu. 1978;13 Pt 2:69-164(Pubmed Abstract)
33. Livingston VW, Alexander-Jackson E. Mycobacterial forms in myocardial vascular disease, J Amer Women’s Assoc 1965; 20:449-452.
34. Dobson RL, Abele DC. Cytologic changes in the eccrine sweat gland in hypothyroidism, J Invest Dermatol 1961; 37:457-458.
35. Cawley EP, Hsu YT, Sturgill BC, et al. Lipofuchsin (“Wear and tear pigment”) in human sweat glands. J Invest Dermatol 1963; 61;105-107.
36. Alexander-Jackson E. The cultivation and morphological study of a pleomorphic organism from the blood of leprosy patients. Intl J Leprosy 1951; 19:173-186.
37. Mattman LH, Tunstall LH, Mathews WW, et al. L variation in mycobacteria. Am Rev Resp Dis 1960; 174:852-861.
38. Mattman LH: Cell wall-deficient forms of mycobacteria. Ann N Y Acad Sci. 1970 Oct 30;174(2):852-61(Pubmed Abstract)
39. Xalabarder C. L-forms of mycobacteria and chronic nephritis. Pub Inst Antituberc 1970;Suppl 7:7-83 (Barcelona).
40. Cantwell AR Jr, Kelso DW, Jones JE. Histologic observations of coccoid forms suggestive of cell wall deficient bacteria in cutaneous and systemic lupus erythematosus.Int J Dermatol. 1982 Nov;21(9):526-37(Pubmed Abstract)
41. Cantwell AR Jr, Cove JK. Variably acid-fast bacteria in a necropsied case of systemic lupus erythematosus with acute myocardial infarction. Cutis. 1984 Jun;33(6):560-7(Pubmed Abstract)
42. Jamieson JD, Palade GE. Specific granules in atrial muscle cells, J Cell Biol 1964; 23:151-172
43. Aplas V. Tierexperimentelle Untersuchungun zur Virusatiologie der Mycosis fungoides. Archiv Klin Exp Dermatol 1963; 205;272-281.
44. Aplas V. Weiterer Beitrag sur Atiologie der Mycosis fungoides. Arch Klin Exp Dermatol 1963; 216:63-70.
45. Cantwell AR Jr. Variably acid-fast pleomorphic bacteria as a possible cause of mycosis fungoides. A report of a necropsied case and two living patients. J Dermatol Surg Oncol. 1982 Mar;8(3):203-13(Pubmed Abstract)
ACKNOWLEDGEMENTS:
Jeanette Volz, Director of the Laboratory of Microtechnique, San Marino, CA, provided histotechnical assistance in this study.
Dan W Kelso, Chief bacteriologist at Central Diagnostic Laboratories, Tarzana, CA, performed the bacterial isolations.
FOOTNOTES
No agency funded this research
Alan R Cantwell, Jr., is the guarantor of this paper
Competing Interests: None Declared
KEYWORDS
eccrine sweat glands
acid-fast bacteria
cell-wall-deficient bacteria
large bodies
scleroderma, linear
lupus erythematosus, cutaneous
leukemia, chronic myelogenous
mycosis fungoides (T cell lymphoma of the skin)
MeSH CLASSIFICATIONS
Sweat Glands
Atypical Bacterial Forms
Transformation, Bacterial
Scleroderma, Circumscribed
Lupus Erythematosus, Cutaneous
Leukemia, Myeloid, Chronic-phase
Mycosis Fungoides
No hay comentarios:
Publicar un comentario